eBioStat ltd. Clinical Trials
www.ebiostat.eu


Description
eBioStat’s main line of business is clinical trials and particularly specializing in Phase IV clinical trials, Pharmacovigilance and post market studies in many diverse areas of medicine, among them Oncology, Diabetes, Anesthesiology, Cardiology and Arthritis using the guidelines, software and methodology of Cambridge University Hospitals Foundation Trust
Services Offered
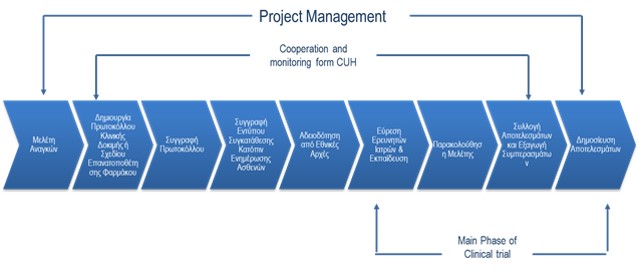
- Protocol Development and/or drug reposition plan
- Protocol writing using CUH writers and patient consent forms
- Protocol Management through the use of CUH methodology and software
- Project Management and licensing from the appropriate public authorities
- Selection, monitoring and evaluation of researchers
- Data analysis and presentation
- Statistical Analysis
- Pharmacovigilance
- Final Report Writing
- Publication of the final report
- Financial management of the trial
In Cooperation With